They continue to undergo continuous and intense safety monitoring. The Program helps COVAX deliver safe and effective COVID-19 vaccines to the high-risk and vulnerable populations in 92 low- and middle-income countries and economies.
Coronavirus Vaccines Leap Through Safety Trials But Which Will Work Is Anybody S Guess
Moderna hopes to have 20 million doses available by the end of 2020 with Pfizer saying that 50 million doses of their vaccine will be available globally by then.
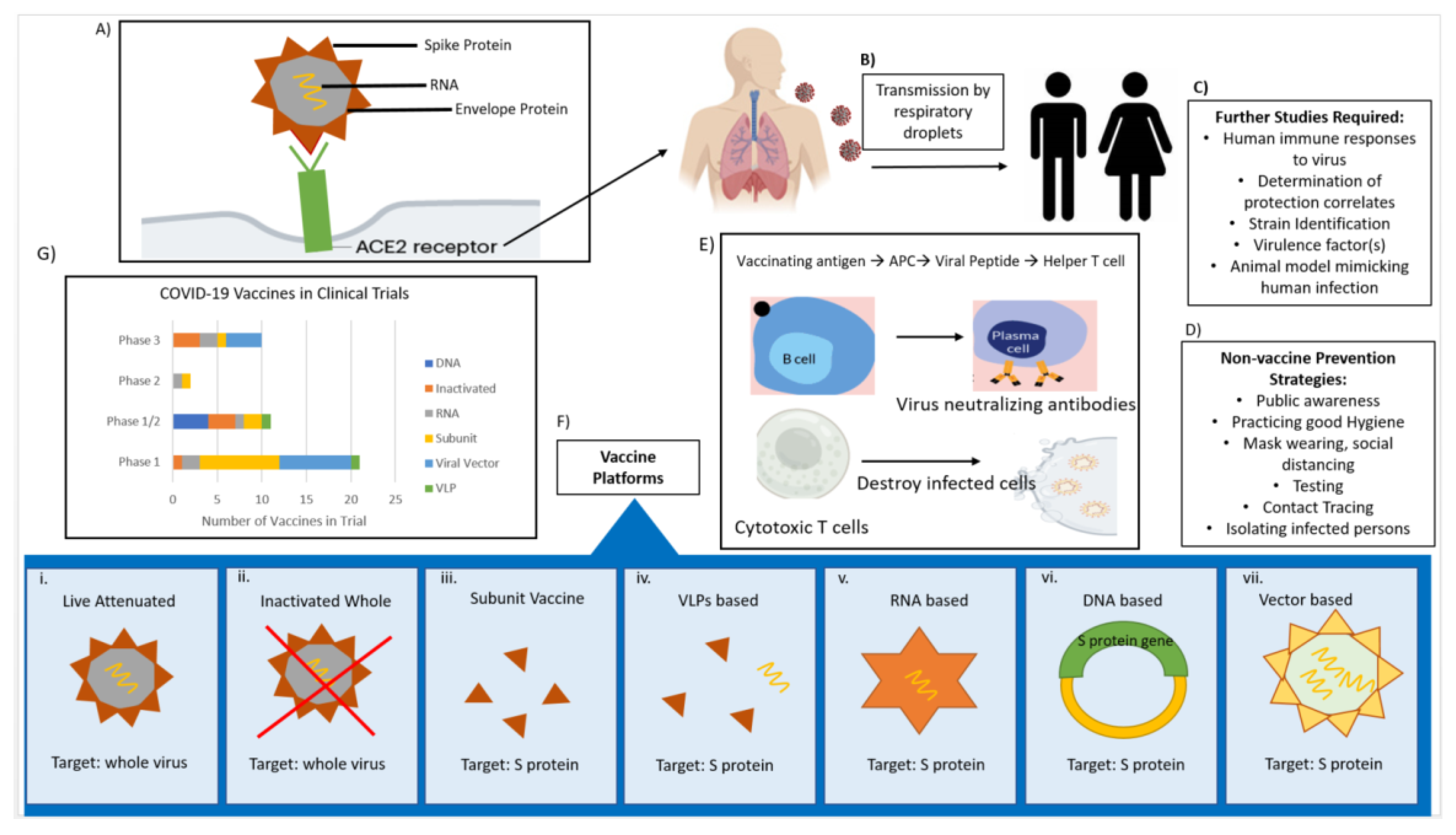
How many vaccine trials for coronavirus. Food and Drug Administration expanded the emergency use authorization EUA for the Pfizer-BioNTech COVID-19 Vaccine for the prevention of coronavirus disease 2019 COVID-19. Typically involving one to several dozen healthy volunteers phase I trials assess short-term safety eg soreness at the site of injection fever muscle aches and immune responses often with different vaccine dosages. Two doses are needed for full protection with the Pfizer and Moderna vaccines while the JJ shot requires a single dose.
Johns Hopkins Medicine is administering all three. A COVID19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 SARSCoV2 the virus that causes coronavirus disease 2019 Prior to the COVID19 pandemic an established body of knowledge existed about the structure and function of coronaviruses causing diseases like severe acute respiratory syndrome SARS and Middle. CDC does not recommend one vaccine.
Only if a vaccine candidate is shown to be safe in. Pfizers trial enrolled over. Covid-19 vaccine One year later many trial participants still to receive certification.
Data from Bloombergs Covid-19 Vaccine Tracker. All three vaccines authorized for emergency use by the Food and Drug Administration FDA have been thoroughly tested and found to be safe and effective in preventing severe COVID-19. The trial demonstrated that the vaccine can protect people from Covid-19 but it left many questions unresolved about the results.
All currently authorized and recommended COVID-19 vaccines. The rapidly growing infection rate of COVID19 worldwide during 2020 stimulated international alliances and government efforts to urgently organize resources to make multiple vaccines on shortened timelines with four vaccine candidates entering human evaluation in March see COVID-19 vaccine Trial and authorization status. Today the US.
Vaccine protection wanes within six months study hints. Different COVID-19 Vaccines. In normal circumstances each step can take two years or more to complete.
The JJJanssen COVID-19 Vaccine was 663 effective in clinical trials efficacy at preventing laboratory-confirmed COVID-19 infection in people who received the vaccine and had no evidence of being previously infected. Reduce your risk of severe illness. If this is the case both companies will have developed and distributed a safe and effective vaccines for COVID-19 in less than a quarter of the time it took to achieve the same for mumpsthe previous record holder.
Hyderabad begins mop-up drive to vaccinate those who have missed out on shots. Are safe are effective and. This on its own is a.
Please see below for more information and safety guidance for the Johnson Johnson COVID-19 vaccine. Do not wait for a specific brand. People had the most protection 2 weeks after getting vaccinated.
A Facebook post states that the Covid-19 vaccines will not have finished testing until 2023. Clinical trials for COVID-19 vaccines were carried out before they were approved by governments and rolled-out to the public. The latest coronavirus news updated every day including coronavirus cases the latest news features and interviews from New.
However for Covid-19 vaccines some of those phases were combined to hasten the process. The post says that therefore the vaccinations are unsafe and suggests that as a result fact checkers have been spreading disinformation regarding the safety of the vaccines. The best COVID-19 vaccine is the first one that is available to you.
The COVAX No-Fault Compensation Program for Advance Market Commitment AMC Eligible Economies is the worlds first and only international vaccine injury compensation mechanism. Following the initial research and development stage a vaccine is first tested on animals and then it goes through three stages of human clinical trials. In particular the post states that studies on the three vaccines currently.

Indonesia To Start Phase Iii Clinical Trials Of Sinovac Covid 19 Vaccine On Tuesday National The Jakarta Post
What Pfizer S Landmark Covid Vaccine Results Mean For The Pandemic
70 Coronavirus Vaccines Are Under Development Who Says Time
Study Oxford Coronavirus Vaccine Induces Immune Response In Early Test Voice Of America English

Trial Of Coronavirus Vaccine Made By Moderna Begins In Seattle The New York Times
Mix And Match Uk Covid Vaccine Trial Expanded Bbc News

Vaccines Free Full Text Efforts At Covid 19 Vaccine Development Challenges And Successes
Fourth Large Scale Covid 19 Vaccine Trial Begins In The United States National Institutes Of Health Nih

Covid 19 Vaccine Trials 9 Things You Should Know Hackensack Meridian Health