As many as. COVID-19 vaccine clinical trials including vaccines in earlier stages of development by visiting clinicaltrialsgov.

Covid 19 Vaccine Research And Development Hillnotes
Six candidate vaccines have been authorized for emergency use or conditional licensed based on their efficacy data in phase 3 trials.

How many covid vaccines in clinical trials. Participants in the clinical trials Across the clinical trials the Pfizer vaccine Comirnaty was studied on about 44000 participants. Only a small proportion of the population in many low-income and middle-income countries will have access to available vaccines. What the trial is looking at.
Participant will be assigned to receive two doses of experimental vaccine or placebo on the schedule of day 014. Tedros Ghebreyesus the Director-General World Health Organisation WHO says a number of vaccines are now in phase three clinical trials to. Phase I clinical trials are the first step in assessing vaccines in people.
104 rows The Vaccines and Related Biological Products Advisory Committee will meet in open session to discuss Emergency Use Authorization EUA of the Janssen Biotech Inc. Clinical trials for COVID-19 vaccines were carried out before they were approved by governments and rolled-out to the public. 1 of 3 claims.
Trial NCT04848584. The FDA continues to review the results of these trials before approving or authorizing COVID-19 vaccines for use. A total of 13000 subjects will be enrolled.
Currently several COVID-19 vaccines are in clinical trials. But because there is an urgent need for COVID-19 vaccines and the FDAs vaccine approval process can take months to years the FDA first gave emergency use authorization to COVID-19 vaccines based on less data than is. AstraZeneca COVID-19 vaccine Novavax COVID-19 vaccine Learn more about US.
COVID-19 Clinical Trials. In particular the post states that studies on the three vaccines. 999 Clinical trial phase.
These trials all aim to compare the safety and. The experimental vaccine and placebo were both manufactured by Sinovac Research Development Co Ltd. Participants of clinical trial of Covid-19 vaccine can download fully-vaccinated certificate.
In just four months the COVID-19 vaccines have killed more people than all available vaccines combined from mid-1997 until the end of 2013 a period of 155 years. A Facebook post states that the Covid-19 vaccines will not have finished testing until 2023. There are currently at least six COVID-19 vaccines in phase 3 clinical trials the final phase of testing.
Clinical researchers work to get COVID-19 vaccine authorized for children under 12 The timeline for a COVID-19 vaccine to be approved for children under the. Clinical trials offer hope for many people and provide an opportunity to help researchers find new or improve existing treatments. Up to now 237 candidate vaccines against SARS-CoV-2 are in development worldwide of which 63 have been approved for clinical trials and 27 are evaluated in phase 3 clinical trials.
As of February 27 2021 large-scale Phase 3 clinical trials are in progress or being planned for two COVID-19 vaccines in the United States. In total 118902 adverse event reports had been filed. Participants had a range of different ethnicities ages sexes and underlying health conditions.
16 and older Number of people all ages. Clinical trials are medical research studies to test new ways to prevent detect or treat diseases. Half of these people received the vaccine and half received a saline placebo.
Typically involving one to several dozen healthy volunteers phase I trials assess short-term safety eg soreness at the site of injection fever muscle aches and immune responses often with different vaccine dosages. As of April 23 2021 VAERS had also received 12618 reports of serious adverse events. The post says that therefore the vaccinations are unsafe and suggests that as a result fact checkers have been spreading disinformation regarding the safety of the vaccines.
Clinical trials use volunteers who agree to participate in these types of studies. Public health Ongoing and future Covid-19 vaccine clinical trials. It is planned that the study will be conducted with two separate cohorts.
Pfizers trial enrolled over. The trial will analyze the effectiveness of two doses of the COVID-19 vaccine at preventing hospitalization from the virusResearchers will also examine its effectiveness against different strains of the virus. Will be issued to those who received both the vaccines during the clinical trials.
COVID-19 Vaccine for active.
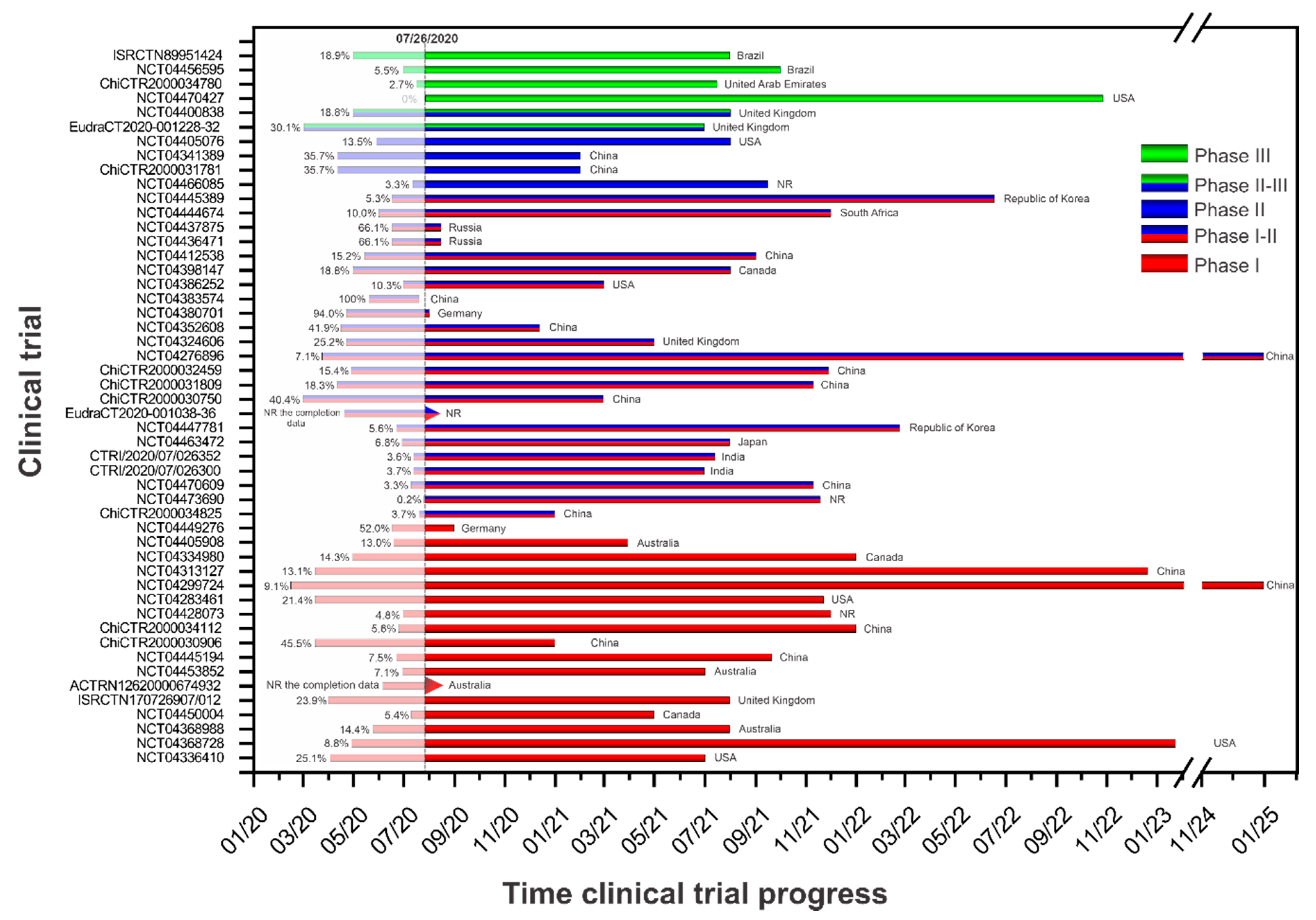
Vaccines Free Full Text Current Clinical Trials Protocols And The Global Effort For Immunization Against Sars Cov 2

Covid 19 Clinical Trials Exhibit Encouraging Results Says Globaldata Globaldata

Public Willingness To Participate In Covid 19 Vaccine Clinical Trials Ppa

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021
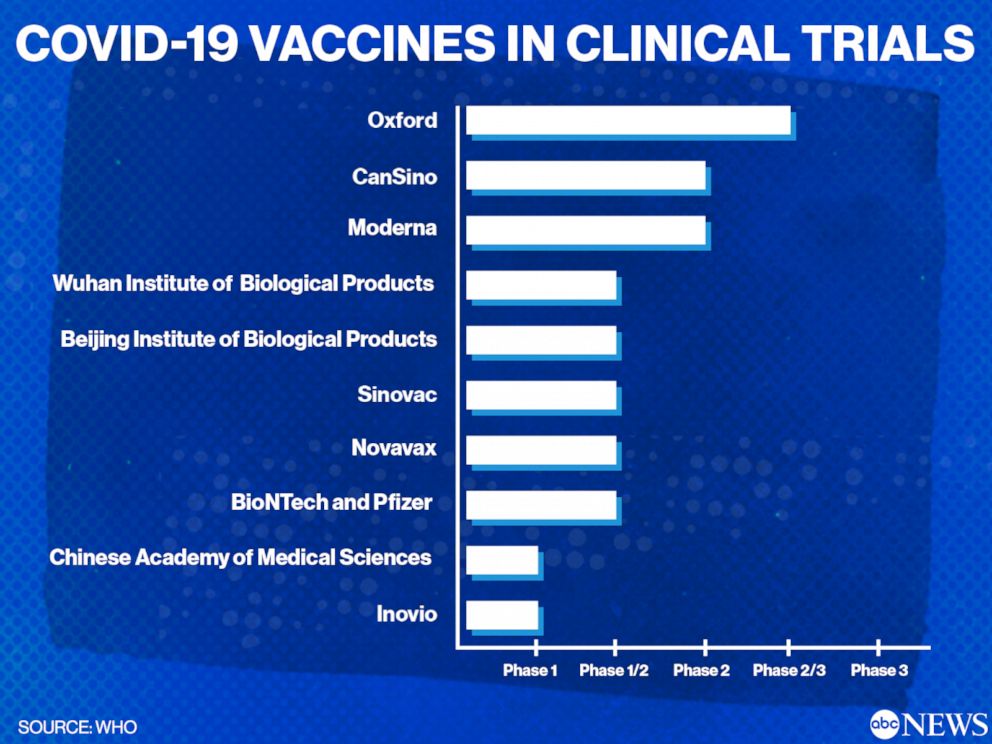
Out Of The Lab And Into People S Arms A List Of Covid 19 Vaccines That Are Being Studied In Clinical Trials Abc News
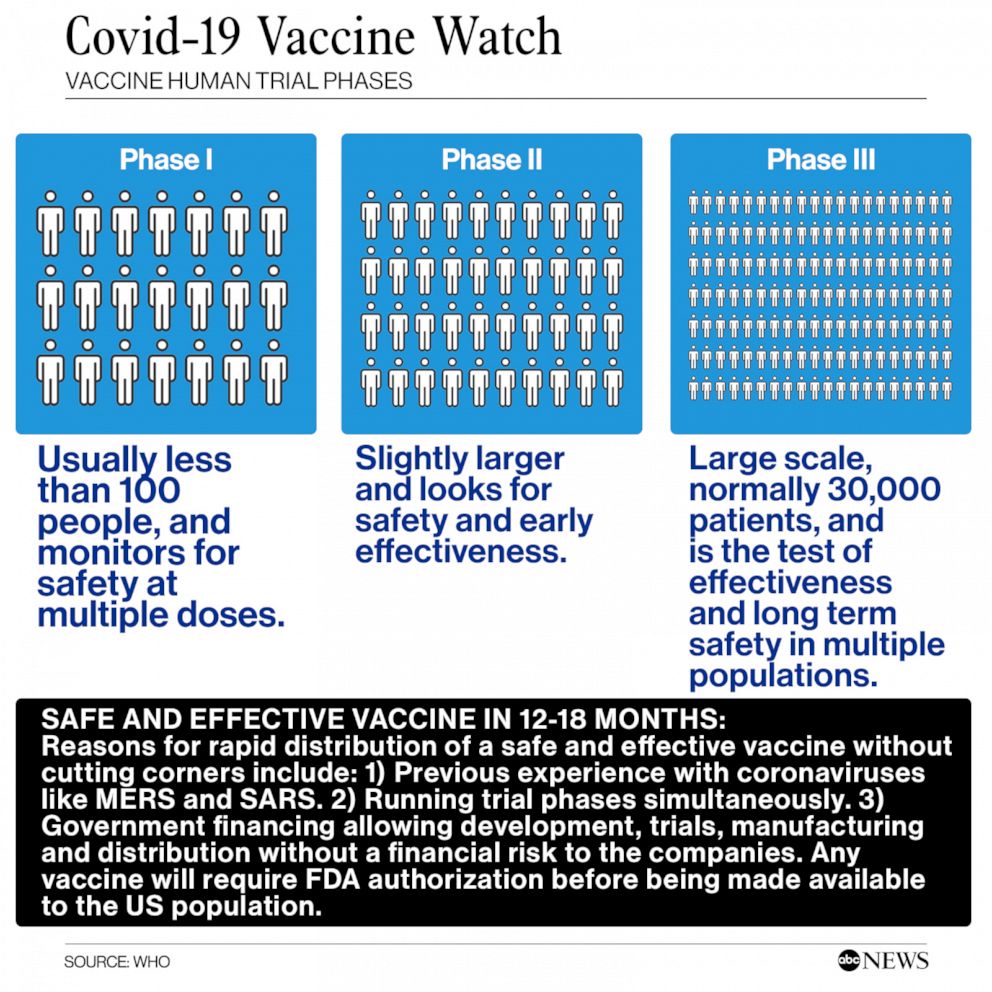
Human Trial For Coronavirus Vaccine Launched By Moderna Enters Phase 3 Abc News

Covid 19 Vaccines A Race Against Time In The Middle Of Death And Devastation Journal Of Clinical And Experimental Hepatology
Chart Covid 19 Vaccine Pipeline Fills Up Again Statista

Covid 19 Vaccines Development Evaluation Approval And Monitoring European Medicines Agency