Find a trial and put your name on the list. The vaccine trial will test to see whether booster vaccines could help protect people against new Covid variants.
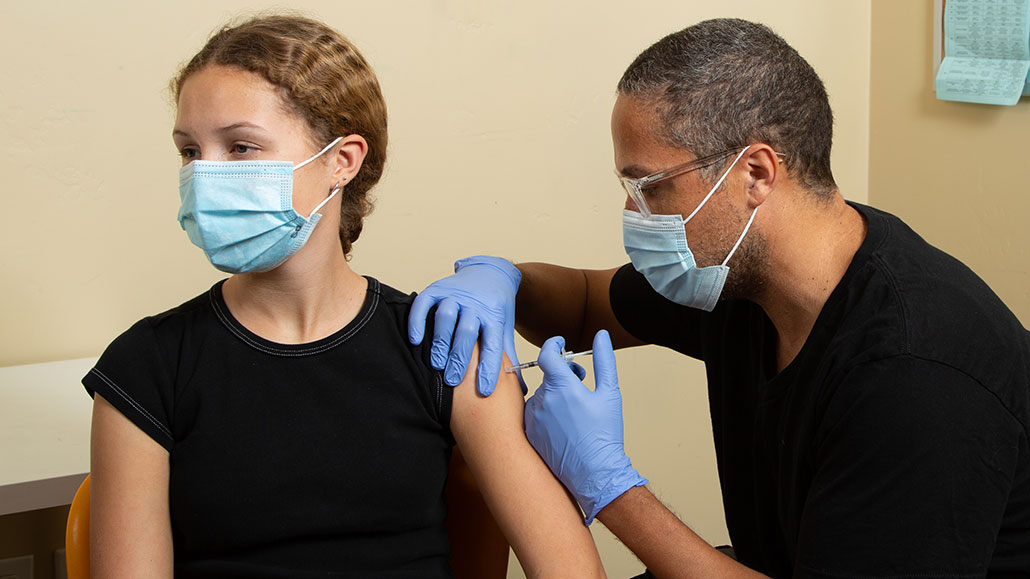
Five Questions About Covid 19 Vaccine Trials In Teens Answered Science News For Students
Now Clinical Trials Management on Houma Boulevard.
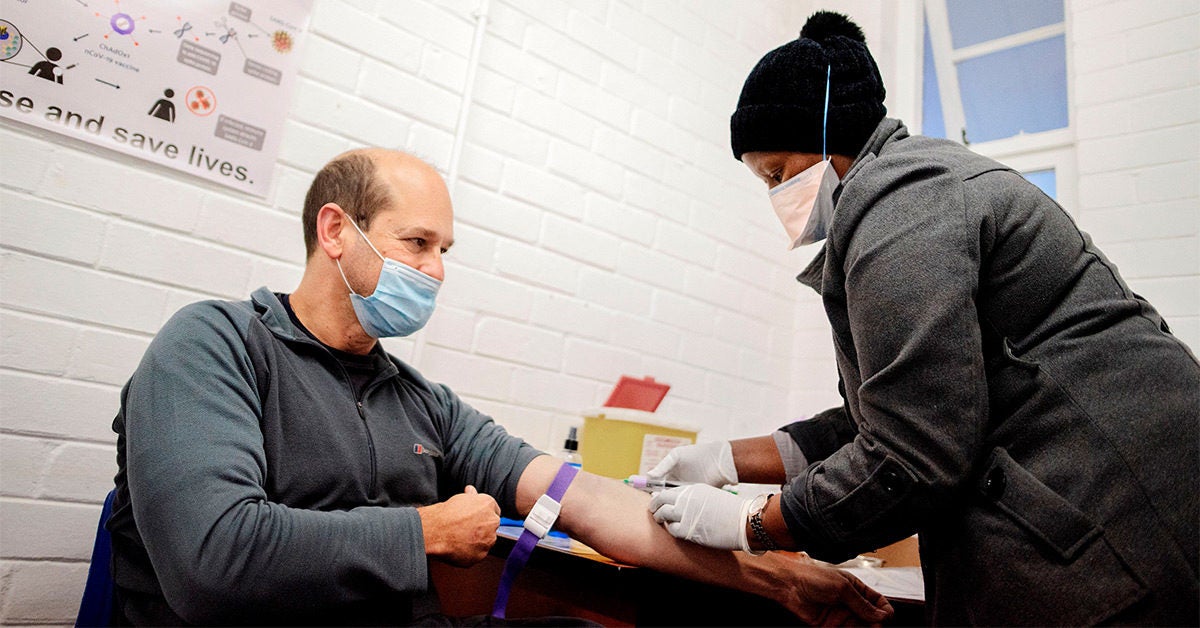
How to sign up for covid vaccine trial. Over 100000 people have volunteered to take part in COVID-19 vaccine trials helping to speed up efforts to discover a safe and effective vaccine. However finding trials that are recruiting participants is more difficult than it sounds. At the Downing Street press briefing on Wednesday the Health Secretary announced the launch of a nationwide study to explore whether giving a third dose booster shot of coronavirus vaccines would be safe and effective in extending immune protection against COVID-19.
How to sign up. The basics are simple. Sign up to Volunteer.
There is no upper age limit. Over 100000 people have signed up for future. The Pfizer-BioNTech COVID-19 vaccine was fully approved on Monday.
When the Johnson and Johnson COVID vaccine was being tested people from the New Orleans area signed up in record numbers for the clinical trial. Its very luck of the draw she says. Researchers often need to include people with different health conditions to see how the vaccine works.
COVID-19 vaccines are 100 free for the patient. In a booster trial that is underway initial data showed that a booster dose which is the third dose of BNT162b2 administered six months following the second dose showed an encouraging tolerability profile. Professor Saul Faust director of the National Institute for Health Research Southampton clinical research facility and lead investigator for the trial said the hope of a booster is that we raise the antibody level enough to be able to cover existing and variant strains of.
The booster vaccine is an updated version of the authorised BNT162b2 Covid-19 vaccine and is made to specifically target the entire spike protein of the Delta variant. Anyone 18 or over living in the UK can sign up to be contacted about taking part in COVID-19 vaccine studies. To see if you may be eligible please email NIAIDCovidVaccineStudyniaidnihgov.
The trial at Rutgers began mid-June and has enrolled about 65 kids so far. If you have a. Families who are interested can call the COVID vaccine clinical trial hotline.
Covid vaccine booster study. How to join a coronavirus vaccine study You can join any of the government-sponsored coronavirus vaccine trials by going to the CoVPN website. We hope to build upon our experience in COVID-19 vaccine clinical trials to partner on this new and innovative vaccine technology he said.
How to Get Your Kid in a COVID Vaccine Trial. Things to know before signing the agreement. You must be legally authorized in your jurisdiction to administer vaccines.
Following adult vaccine trials the FDA asks for two months worth of follow-up safety data but for the childrens trials. Study of How COVID-19 Affects Blood Cells and the Immune System. USC Clinical Trials Director Lucas Litewka said the need for new and continuing research into potential COVID-19 vaccines was fundamental to improving our pandemic response and preparedness.
Reports should include the words Moderna COVID19 Vaccine EUA in the description section of the report. To be a vaccination provider your health system or you as an independent provider are required to sign the CDC COVID-19 Vaccination Program Provider Agreement. To study how SARS-CoV-2 the virus that causes COVID-19 affects the body specifically how it affects blood cells and the immune system.
Banerjee has been trying for months but to no avail. Report to ModernaTX Inc. Indias First mRNA COVID Vaccine Candidate Found To Be Safe Gets DCGI Nod For Phase 23 Clinical Trials by Swarajya Staff - Aug 25 2021 0530 AM Covid-19 Vaccine.
Moderna whose vaccine currently is only available for adults began recruiting kids for its pediatric vaccine trial in March. By collecting details about people who are interested in taking part in vaccine studies the service will help cut down the time it takes to find volunteers for vaccine studies. Applications will open shortly via the studys website.
You can sign up to give permission for researchers to contact you about taking part in COVID-19 vaccine studies. 631-638-COVI and leave a message with their name their childs name and date of birth and a. How to sign up for clinical trial.
People interested in volunteering can sign up via the NHS Vaccine Research Registry where theyll be asked to answer some questions about themselves and give permission to. In this region Moderna is recruiting participants for a site at the Childrens Hospital of Philadelphia. To be eligible for the study you must be aged 30 and over and have received both doses of the Covid-19 vaccine.
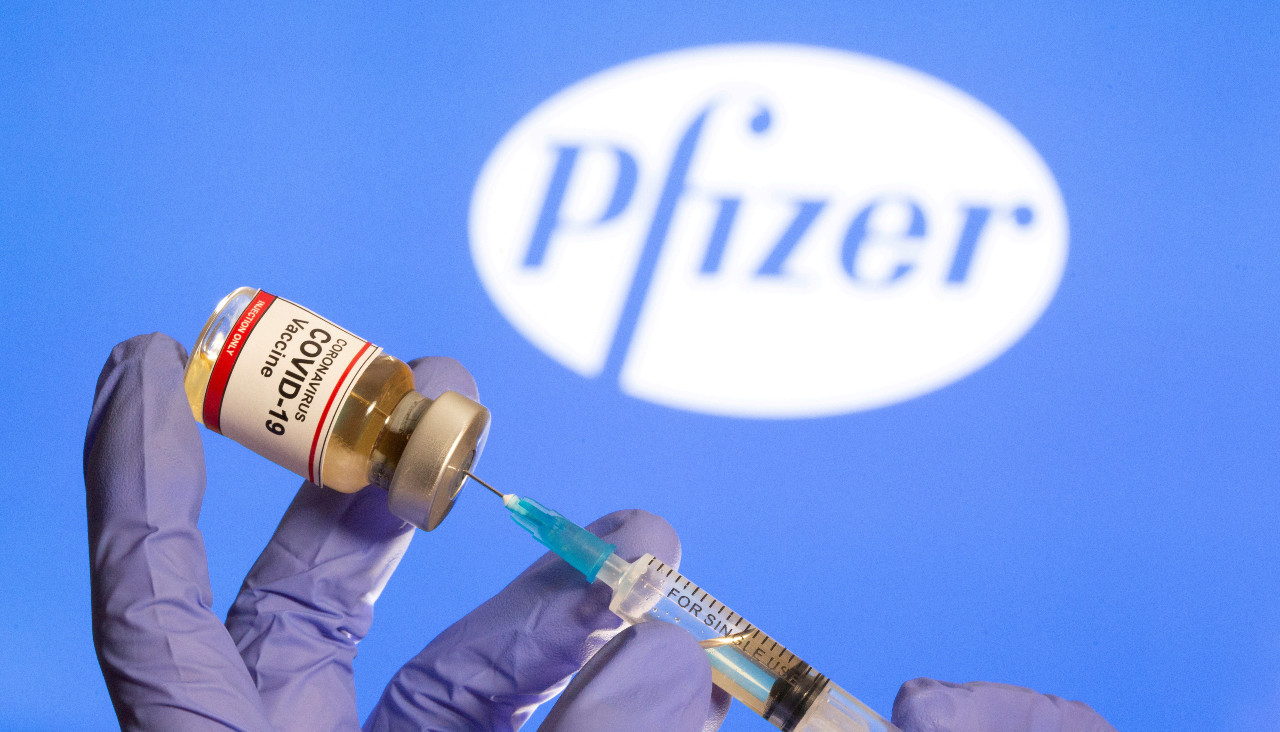
Pfizer Vaccine Trial Success Signals Breakthrough In Pandemic Battle World The Jakarta Post
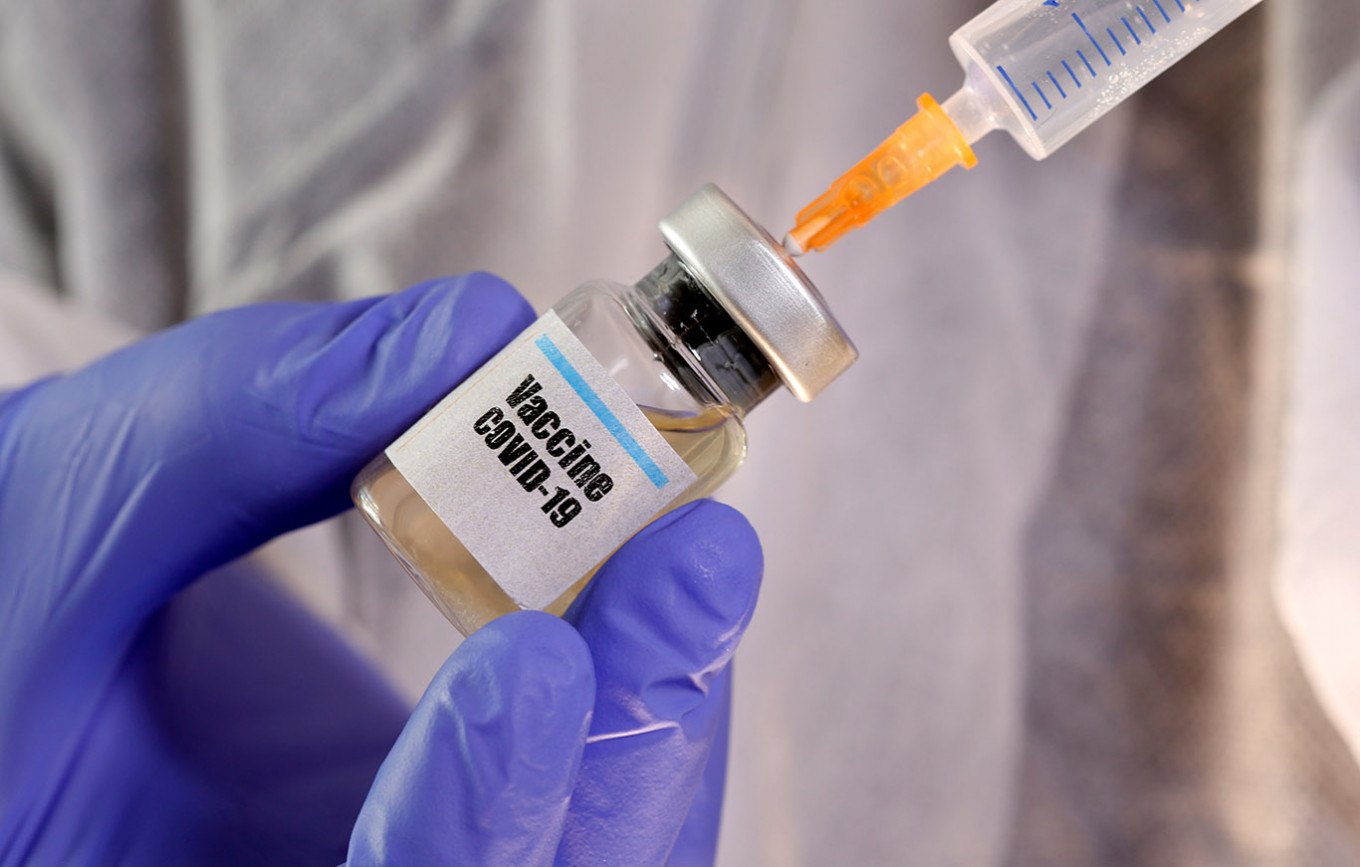
German Chinese Coronavirus Vaccine Trial Begins In China World The Jakarta Post
70 Coronavirus Vaccines Are Under Development Who Says Time
Early Clinical Trial Data In For Whole Virus Covid 19 Vaccine Candidate
We Need One Million Volunteers For Covid 19 Vaccine Trials Time

Thousands Sign Up For Covid 19 Vaccine Clinical Trials In France

Trial Of Coronavirus Vaccine Made By Moderna Begins In Seattle The New York Times
Mix And Match Uk Covid Vaccine Trial Expanded Bbc News

Covid 19 Vaccine Trial Volunteers Explain Why They Signed Up